BRC News & Events
– 2025 –

Dr. Tristan McIntosh
Dr. Alison Antes
NEW PAPER ON LEADERSHIP, MANAGEMENT, AND MENTORING PUBLISHED IN FRONTIERS
- The necessity of LMM in research settings
- Defined roles and responsibilities of research leaders, managers, and mentors
- Behaviors, skills, and practices necessary for effective performance in LMM roles
- Challenges and solutions in LMM roles
- Evaluation methods including top-down, bottom-up, and self-evaluation

QDS TEAM PUBLISHED IN THE INTERNATIONAL JOURNAL OF QUALITATIVE RESEARCH
“Responsible Sharing of Qualitative Research Data: Insights from a Pioneering Project in the United States,” written by James DuBois’ Qualitative Data Sharing (QDS) team, was recently published in the International Journal of Qualitative Research. Sharing qualitative data promotes open science, reduces research costs, and improves transparency. Despite mandates from institutions like the NIH, researchers have concerns about QDS due to its sensitive nature. In the article, the team details how they worked to identify and reduce barriers to QDS. As part of their project, they developed guidelines and tools for data de-identification, depositing, and sharing. They also developed and tested the Qualitative Data Sharing (QuaDS) Software to support qualitative data de-identification. In the article, the authors describe the process of recruiting, enrolling, and assisting 28 researchers to use the guidelines and software to prepare and de-identify data for deposit in a repository. The team ultimately demonstrated that QDS is feasible, but the article highlights the need for clear guidelines, support, and improved processes.

CNN HIGHLIGHTS THE IMPORTANT WORK OF THE BRC’S DR. JESSICA MOZERSKY
Dr. Jessica Mozersky, along with a team of researchers led by Dr. Sara Hartz, a professor of psychiatry at WashU Medicine, evaluated data from patients being treated with one of two novel disease modifying therapies for Alzheimer’s disease, lecanemab and donanemab. Both drugs are antibody therapies that help clear plaque-causing amyloid proteins from the brain.
The purpose of the study was to translate the clinical trial results into accessible, meaningful information so that patients and caregivers can make informed decisions regarding treatments and carefully weigh the risks and benefits. “What people want to know is how long they will be able to live independently, not something abstract like the percent change in decline,” said Dr. Hartz.
The researchers found that a typical person with very mild symptoms could expect to live independently for another 29 months without treatment, 39 months with lecanemab, and 37 months with donanemab. Typically a person with mild, rather than very mild, symptoms is no longer able to live independently. For these individuals, researchers determined that they could expect to manage self-care independently for an additional 26 months if treated with lecanemab and 19 months with donanemab.
The findings of this study, “Assessing the clinical meaningfulness of slowing CDR-SB progression with disease-modifying therapies for Alzheimer disease,” were published in Alzheimer’s & Dementia: Translational Research & Clinical Interventions on February 13. Following publication, CNN interviewed Dr. Hartz for the story, “How much longer you might live without assistance on new Alzheimer’s drugs.”
– 2024 –

GREENWALL FOUNDATION AWARDS BAKER WITH 2-YEAR MAKE A DIFFERENCE GRANT
The BRC’s Dr. Lauren Baker has been awarded a two-year grant by the Greenwall Foundation to fund her study, “Psychotropic Medication Decision Making for Youth in Foster Care: Challenges, Barriers, and Facilitators to Informed Consent, Assent, and Refusal.” In this study, Dr. Baker and the research team aim to examine the challenges, barriers, and facilitators to informed consent, assent, and refusal in treatment decisions regarding psychotropic medications for youth in foster care. Through analysis of state child welfare policies, foster care bills of rights, and qualitative interviews with foster care alumni, case workers, and clinicians, the team will develop a set of best practice and policy recommendations to foster ethical decision-making related to psychotropic medication for foster care youth.

ANTES APPOINTED AS NEW OMSBUD
Alison Antes, PhD, associate professor of medicine in the Bioethics Research Center and Division of General Medicine and Geriatrics, has been appointed as one of two new ombuds for Washington University School of Medicine in St. Louis. Antes was appointed alongside Mwiza Ushe, MD, MA, professor of neurology. Their terms begin Jan. 1, 2025.
The goal of the Office of the Ombuds is to meet and provide a confidential space for faculty, WashU Medicine students, residents, fellows and postdocs to “voice concerns, develop options and consider ways to solve problems.” The office also reports anonymized statistical data and complaint patterns to university officials so appropriate system-wide action can be taken.
Read full announcement here.
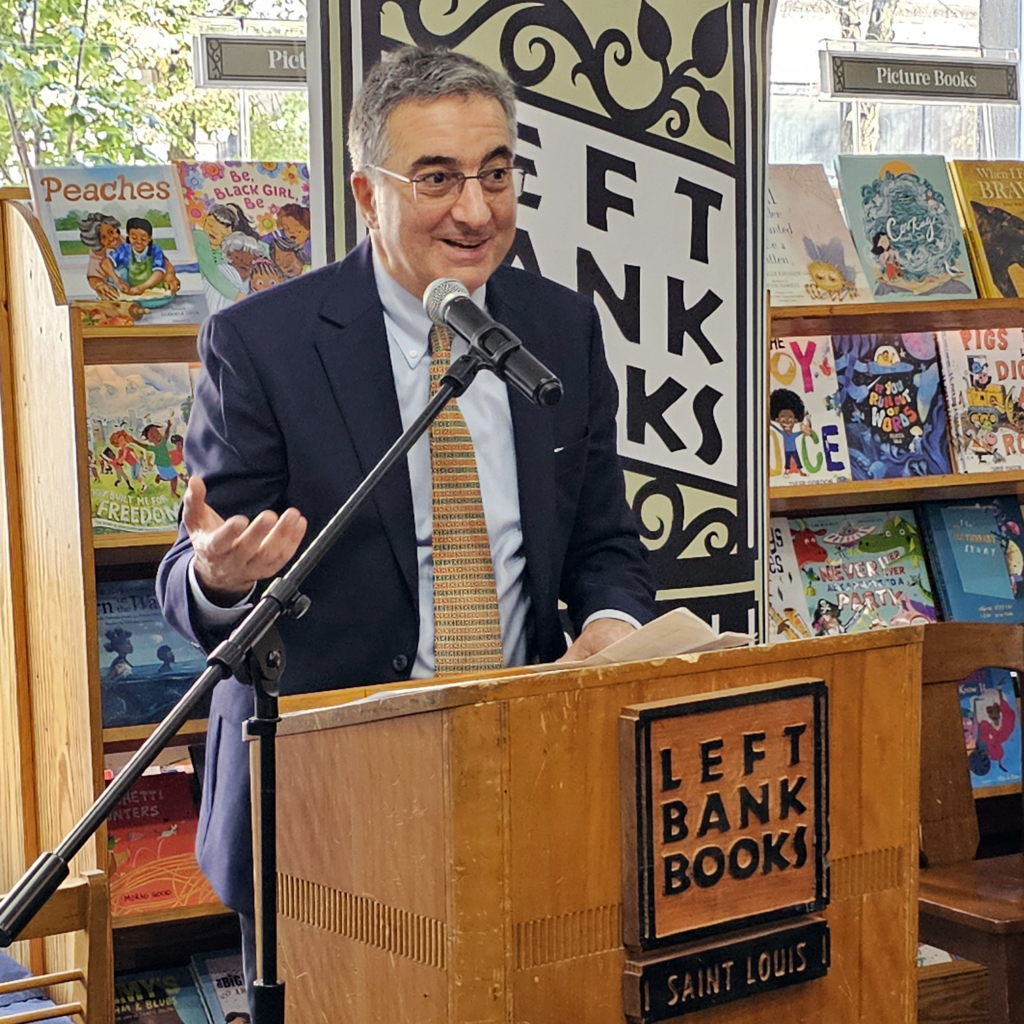
THE BRC HOSTS AUTHOR EVENT WITH DR. ROBERT KLITZMAN
On September 18, 2024, the BRC partnered with Left Bank Books to welcome Dr. Robert Klitzman for an author talk and book signing. His latest book, “Doctor, Will You Pray for Me?: Medicine, Chaplains and Healing the Whole Person,” is an in-depth exploration of the fascinating world of hospital chaplains.
Dr. Klitzman is a Professor of Psychiatry and the Director of the Masters of Bioethics Program at Columbia University. He has authored nine books and more than 150 scientific articles, as well as essays in the Wall Street Journal, Newsweek and the Nation. He regularly contributes to the New York Times, CNN and elsewhere.
Chaplains, bioethicists, and others who find Dr. Klitzman’s works fascinating attended this event in St. Louis’ Central West End neighborhood. Following his talk, Dr. Klitzman signed copies of his latest book and chatted with guests. This event was supported by the BRC’s research program, “Healthcare, Values and the Spiritual Life.”

MWOBOBIA RECOGNIZED FOR HIGHEST-SCORING ABSTRACT
Judith Mwobobia, MS, a graduate research assistant at the BRC working on a NIH BRAIN Initiative project led by BRC faculty Tristan McIntosh, PhD, submitted a proposal to present at the American Public Health Association Annual Meeting (Student Conference). Mwobobia’s synopsis, “Navigating Ethical Frontiers in Neurotechnology Partnerships,” has been recognized with the APHA Ethics Section 2024 Award for highest-scoring abstract for a student conference proposal. Mwobobia will travel to Minneapolis, MN at the end of October to present project findings from in-depth interviews with neuroethicists about industry-academia partnerships in the research and commercialization of brain technologies.

MCINTOSH AWARDED FOR “OUTSTANDING TALK”
Tristan McIntosh, PhD, Assistant Professor of Medicine at the BRC, was recognized for her presentation at the International Neuroethics Society annual meeting on Wednesday, April 17 with an “Outstanding Talk” award. At this forum, she presented her research on “Risk factors and ethical considerations for developing and commercializing neurotechnologies: Findings from interviews with institutional officials.” Congratulations Dr. McIntosh!

MCINTOSH TAPPED FOR STATE MEDICAL BOARDS EXPERTISE IN MAY 2024 ISSUE OF MEDICAL ETHICS ADVISOR
-
Implementing a screening committee to triage incoming complaints, and
-
Providing consistent and complete disciplinary information to national databases

DR. JESSICA MOZERSKY RECEIVES THE LAURA S. AND JOHN A. BLUMENFELD AWARD
Jessica Mozersky, PhD, MBE, Assistant Professor of Medicine was nominated by Matt Gabel, PhD and Sarah Hartz, MD, PhD for the Laura S. and John A. Blumenfeld Award. This award recognizes a highly promising early-stage investigator at Washington University in St. Louis who is pursuing an academic career that focuses on Alzheimer disease and related dementia (ADRD). Mozersky received a $3,000 monetary gift to further personal development goals. The Blumenfeld Family Education Award is intended to foster the next generation of ADRD investigators. The Blumenfeld family and others gathered on March 27, 2024 at the Knight ADRC to honor Mozersky, as well as the recipient of the Richard and Mildred Poletsky Award.

CLINICAL JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY PUBLISHES BRC ARTICLE REPORTING CONSENSUS PANEL RECOMMENDATIONS
Recently, the Clinical Journal of the American Society of Nephrology published an article with recommendations from a Delphi Consensus Panel that evaluated ApoL1 genetic testing options for kidney transplant centers. This gene can increase the chances of getting kidney disease, especially in people with African ancestry. Different transplant centers have different ways of testing for this gene, but there are no set rules on who should be tested and how the results should be used.
A group of people served on the consensus panel, including kidney donors, family members of deceased donors, kidney recipients, genetic counselors, surgeons, and transplant doctors. Most panelists were Black or African American, which is important because the risky gene is mostly found in people with African ancestry. They discussed things like who should be tested, who gets to decide whether someone is allowed to donate an organ, and how the test results should be used.
They supported some ApoL1 policies and disagreed with others. For example, they agreed that instead of asking about race, it’s better to ask about ancestry. They also agreed that donors should be told about the results and encouraged to share them with family and other people involved in the transplant process. They panel thought that doctors should use the results together with the donors to make decisions. Transplant centers should not make these decisions on their own. Find the full article with the complete findings here.
McIntosh, T., Walsh, H., Baldwin, K., Iltis, A., Mohan, S., Sawinski, D., Goodman, M., & DuBois, J. M. (2024). Evaluating ApoL1 Genetic Testing Policy Options for Transplant Centers: A Delphi Consensus Panel Project with Stakeholders. Clinical Journal of the American Society of Nephrology. https://doi.org/10.2215/CJN.0000000000000397
DR. SISK RECEIVES PHILANTHROPIC GIFT TO DEVELOP A NETWORK OF CLINISCIANS TREATING VASCULAR ANOMALIES
Dr. Bryan Sisk has been gifted nearly $700,000 to develop and study the implementation of a novel program called VACcess. The goal of this program is to develop a collaborative network to support clinicians who care for patient with vascular anomalies. Vascular anomalies are caused by abnormal overgrowth of blood vessels that can lead to pain, disfigurement, disability, and even death. Because these disorders are rare, many patients struggle to find clinicians who are familiar with these disorders. As a result, these patients are often misdiagnosed, receive inappropriate treatments, and experience poor medical care. These problems are even greater for adults with vascular anomalies.
With this philanthropic gift, Dr. Sisk will develop VACcess, a collaborative network of clinicians that will offer expert medical guidance to clinicians caring for patients with vascular anomalies. The program will (1) create a network of specialists willing to provide guidance to clinicians without expertise in vascular anomalies, (2) provide consensus responses to questions asked by clinicians about vascular anomalies, (3) provide guidance on referrals to vascular anomaly clinics, and (4) review especially complicated cases in depth during remote case conferences.
This program will be the first of its kind in vascular anomalies. In the future, VACcess can serve as a model for nearly 7,000 other rare diseases in which patient have similar barriers to high-quality care.
– 2023 –

RECENT PUBLICATIONS
The paper, “Exchanging words: Engaging the challenges of sharing qualitative research data,” was recently published in Proceedings of the National Academy of Sciences (PNAS). In January 2023, a new NIH policy on data sharing – both quantitative and qualitative research (QR) – went into effect. In this perspective article, the research team shares important lessons learned by addressing eight clusters of questions on issues such as where, when, and what to share; how to deidentify data and support high-quality secondary use; budgeting for data sharing; and the permissions needed to share data. We also offer a brief assessment of the state of preparedness of data repositories, QR journals, and QR textbooks to support data sharing.
DuBois, J. M., Mozersky, J., Parsons, M., Walsh, H. A., Friedrich, A., & Pienta, A. (2023). Exchanging words: Engaging the challenges of sharing qualitative research data. Proceedings of the National Academy of Sciences (PNAS), 120(43). www.pnas.org/doi/10.1073/pnas.2206981120

TEAM FROM BIOETHICS RESEARCH CENTER AND THE INSTITUTE FOR INFORMATICS HELP RESEARCHERS SHARE QUALITATIVE DATA
James DuBois and a team of researchers in the Bioethics Research Center and the Institute for Informatics are helping researchers to share data that have traditionally remained hidden. While numeric data are commonly shared, data from interviews and focus groups have rarely been shared because they often contain sensitive information and are difficult to de-identify. This is about to change. In January 2023, the National Institutes of Health’s new policy on data sharing went into effect, requiring all research data to be shared. Data sharing increases trust in science, supports new research with existing data, and provides opportunities for students to learn research methods with real-world data.
With funding from the National Human Genome Research Institute, the Wash U team pioneered qualitative data sharing in the US. Data were shared by 29 researchers who had conducted research interviews and focus groups. As part of the project, the Wash U team has developed and recently licensed software that helps de-identify interview text. They also developed a qualitative data sharing toolkit (QDStoolkit.org), which provides guidance to researchers. In October, the Proceedings of the National Academies of Science (PNAS) published a paper sharing key lessons learned in this 5-year project. This paper helps researchers to answer the questions: What should be shared, when, and where? What permissions do I need to share data? How do I de-identify data? This last question has vexed researchers because manual de-identification of texts is time-consuming and challenging. The team’s qualitative data sharing (QuaDS) software has performed very well in flagging potentially identifying information, and will be commercially available in early 2024 through the Institute for Mixed Methods Research (immrglobal.org).
Visit qdstoolkit.org to learn more about this work.

COMPASS TRAINING AND MENTORING PROGRAM SEES MASSIVE GROWTH IN FIRST YEAR
BRC faculty, Drs. Alison Antes (Director) and Tristan McIntosh (Co-Director) continue to grow their NIH-funded Compass training and mentoring program to develop skills in leadership and management (L&M) for early-career researchers and late-stage postdocs. Developed by a team of researchers, workplace psychologists, and career development experts, Compass provides guidance and tools to help program scholars be intentional about leading people and managing scientific work. Scholars learn from one another and experienced mentors in a supportive community, Compass believes the keys to successfully running an independent lab should be accessible to all. To ensure equitable access to the training needed to find early-career success, Compass is open to biomedical researchers across the United States and is free for the duration of the grant.
Demonstrating how much this training is needed, Compass has grown from 24 scholars in the inaugural fall 2022 cohort, to 74 scholars this past spring, to over 150 scholars participating in the third cohort this fall. Compass is actively recruiting for the spring 2024 cohort and reviews applications on a rolling basis. You can learn more about the program and apply on the Compass website at https://researchercompass.org.
Compass is supported by a five-year IPERT (Innovative Programs to Enhance Research Training) grant through the National Institutes of Health (“Program for Advancing Early-Career Researcher Excellence through Leadership and Management Practices” Grant Number R25GM143346).

DR. MOZERSKY RECEIVES FUNDING TO EXAMINE THE IMPACT OF PAYMENT ON RECRUITMENT FOR ALZHEIMER DISEASE RESEARCH
Dr. Jessica Mozersky, Director of Consultation and Assistant Professor of Medicine at the BRC has received funding for a one-year developmental project from the Knight Alzheimer Disease Research Center (Knight ADRC) at Washington University. The project examines the impact of payment on recruitment to Alzheimer Disease (AD) research. Recruitment of participants into AD research, especially of historically underrepresented individuals, is a national priority. There is a lack of empirical evidence on whether payment may increase recruitment. Using a randomized design, we will determine whether discussing payment during outreach and screening increases interest and recruitment into research at the Knight ADRC. Dr. Mozersky and Dr. Matthew Gabel, professor of Political Science at Washington University, are co-leading the project.

DR. ANTES NAMED TO HONOR ROLL

CONTINUING ED: MEMBERS OF THE BRC ADVANCE THEIR EDUCATION
The BRC is pleased to recognize three staff members who have recently earned certifications and advanced degrees. Joe Grailer joined the BRC in 2022 and has since earned his Project Management Professional (PMP) certification from the Project Management Institute. Meredith Parsons, who has served as a research staff member at the BRC since 2017, has earned a Master’s of Science in Applied Health Behavior Research (AHBR) from Washington University School of Medicine. Lauren Baker joined the BRC as a Staff Scientist in 2022. This month, she completed her PhD in Health Care Ethics from Saint Louis University. Congratulations to these team members on their accomplishments!
– 2022 –

THE P.I. PROGRAM – FILLING A VOID IN ACADEMIA
The Professionalism and Integrity Program (P.I. Program) fills a void in academia. Today’s research environments are complex. Researchers are busy, research is heavily regulated, and funding is highly competitive. The P.I. Program is led by Drs. James DuBois and Alison Antes at Washington University. It helps researchers who have intentionally or unintentionally violated research rules or regulations. The program teaches decision-making skills, good lab leadership and management practices, and basic rules-of-the-road for the responsible conduct of research. The program helps researchers do the work they are passionate about while developing efficient approaches to ensure research compliance and integrity. To hear one participant’s experience and remarks from an institutional official, check out this article in Outlook Magazine, published by Washington University School of Medicine – St. Louis. For more information, visit www.integrityprogram.org.

DRS. MOZERSKY AND HARTZ DEVELOP ALGORITHM TO DETERMINE RISK OF ALSHEIMER’S DISEASE
Jessica Mozersky, PhD, assistant professor of medicine at the BRC, and co-principal investigator Sarah Hartz, MD, PhD, an associate professor of psychiatry at Washington University School of Medicine, have developed an algorithm that can help provide people who volunteer for studies of aging with information about the absolute risk each faces of developing dementia due to Alzheimer’s disease. Their findings were recently published in the Journal of Alzheimer’s and Dementia. “We developed the algorithm because study participants wanted more than just a report of whether their test results were normal or abnormal,” Mozersky said. Learn more about their findings here.

THE BRC’S TRISTAN MCINTOSH, PhD CO-EDITS SYMPOSIUM ON RECEIVING THE GIFT OF LIFE
A narrative approach to ethics and healthcare recognizes that listening to stories is crucial for good patient care. Since its inaugural issue in 2011, the journal Narrative Inquiry in Bioethics (NIB) has published stories in its narrative symposia section written by patients, family members, and healthcare providers focused on healthcare and clinical research experiences. The stories in NIB and the accompanying commentaries written by experts in the field, encourage listening, spark empathy, and stimulate discussion on important topics in medical and research ethics.
In its latest symposium, “Receiving the Gift of Life: Stories from Organ Transplant Recipients,” published in Volume 12, issue 2, NIB continues its mission with a look into the experiences of those who have received an organ transplant from a living or deceased donor. The symposium was co-edited by the BRC’s Tristan McIntosh, Ph.D. and Jason T. Eberl, Ph.D. of the Albert Gnaegi Center for Health Care Ethics at Saint Louis University.
Organ transplantation can extend an individual’s life by decades. It can also greatly improve an individual’s quality of life. At the same time, organ transplantation is expensive and stressful. Recipients can experience complications, including organ rejection, and there are few appealing alternatives.
“The purpose of this issue is to learn from the stories of organ recipients in order to tease apart various moral assumptions, gaps, wrongs, insights, and experiences,” McIntosh and Eberl explain. “The stories attend to the details of networks of care, the interplay between donor families and organ recipients, the arduous journey to transplantation (and sometimes re-transplantation), and conflicts of medical and personal values. They also highlight themes of resilience, technology, gift-giving and receiving, sense of self, and moral responsibility.”
Once transplanted, many recipients will have complex health and practical support needs. These could include medication sorting, watching for signs of infection or rejection, as well as requiring help with activities such as bathing, cooking, and wound care. In their introduction to the symposium, McIntosh and Eberl relay that “Public narratives of organ transplantation usually show recipients as fully-recovered with no limitations to their lives or lingering health issues, often including expressions of gratitude. Organ transplantation is thus seen as a miraculous rebirth back into health without frailty. […]. Most people are unaware that, even after transplantation, health management is still challenging, and sometimes comes with lingering chronic conditions.”
Three commentary articles, written by Macey Leigh Levan, Heather Lannon, and Vidya A. Fleetwood, Roslyn B. Mannon & Krista L. Lentine, offer important insights into the authors’ stories. Publication of the issue was made possible through funding from Mid-America Transplant, the UCSF Lung Transplant Program, Donor Connect, William L. Freeman, MD, MPH, MJIL, CIP, Janet Monk, and several individuals who gave anonymous donations. An open-access edition of the symposium and an accompanying teaching guide are in production. Both resources will be available on the NIB website under the VOICES tab in early Winter 2023.

BRC RECEIVES NEW R01 GRANT FROM NIH
James DuBois, the Executive Director of the BRC, has received a $1.5 million 3-year grant from the National Human Genome Research Institute for a project called, “Religion and support for genomic healthcare: An exploratory study of the US public and faith leaders.”
Religion is a primary driver of concerns with genetic medicine, which includes activities such as prenatal genetic testing, gene editing, and the development of mRNA vaccines. Ninety percent of the US public believes in some kind of higher power and 55% pray daily. Most health disparity populations are more highly religious than the general US public. This project seeks to understand why higher levels of religious practice are associated with greater concerns with genetic medicine.
Additionally, this project will interview faith leaders to identify how genetic medicine might engage faith communities in ways that are respectful and constructive. While some concerns with healthcare technologies may disappear with new information, others stem from worldviews, deep moral commitments, or mistrust of the healthcare system. Very few models exist for engaging faith communities about genetic medicine, particularly models that directly address religious and moral concerns.
This will be the first major project of the “Healthcare, Values, and the Spiritual Life” research program. To learn more about this program, please visit the HVS website.

BRC WELCOMES GRADUATE RESEARCH ASSISTANTS
The BRC is pleased to welcome Graduate Research Assistants Elizabeth Bingheim, Gary Liu, and Minglu Liu. Under the guidance of Dr. Alison Antes, Elizabeth and Minglu will be supporting the R25 project, “Program for Advancing Early-Career Researcher Excellence through Leadership and Management Practices.” Gary Liu will be working on a Saint Louis University Bander Center datafication and healthcare collaborative.

BRC WELCOMES LAUREN BAKER, MA, PHD(c)
Lauren Baker has joined the Bioethics Research Center as a Staff Scientist. Currently, she is a doctoral candidate in health care ethics at Saint Louis University. Prior to joining the BRC, she taught undergraduate classes in bioethics and public health ethics at Saint Louis University. Her work has appeared in academic journals such as Theoretical Medicine and Bioethics, the American Journal of Bioethics, and the Journal of Comparative Effectiveness Research.

BRC WELCOMES MADI ENLOE, MPH, BSN, RN
Madi Enloe has joined the Bioethics Research Center in the Division of General Medical Sciences as a Senior Public Health Research Technician. She earned dual bachelor’s degrees in public health and nursing from Saint Louis University, and a master’s degree in public health from Saint Louis University. Madi comes to the BRC from healthcare and will be supporting research projects led by Dr. Bryan Sisk.

BRC RECEIVES R01 GRANT FROM NIH BRAIN INITIATIVE FOR 4-YEAR PROJECT
BRC faculty Dr. Tristan McIntosh received funding through the National Institutes of Health BRAIN Initiative to support a 4-year project: Fostering Ethical Neurotechnology Academia-Industry Partnerships: A Stakeholder Engagement and Toolkit Development Project.
Industry-academia (IA) partnerships are critical for bringing neurotechnologies (e.g., implants) to market for public benefit. Neurotechnologies present unique ethical challenges compared to other health innovations: they can alter brain function and record and transmit brain activity.
This project addresses a gap in our understanding of ethical complexities inherent to IA neurotechnology partnerships. By engaging relevant stakeholders (e.g., industry, academic researchers, university officials, patients, ethicists), we will work to identify promising solutions and actionable guidance for navigating competing priorities, mitigating risk, and balancing scientific values with financial goals. We will use this knowledge to develop and disseminate an innovative, publicly accessible toolkit to help stakeholders cultivate ethical and responsible industry-academia partnerships.

DR. ANTES INVITED TO WRITE OP-ED IN SCIENTIFIC AMERICAN
Eric Lander, Director of the Office of Science and Technology Policy (OSTP) and top White House science adviser, stepped down on February 7, 2022. This resignation followed the conclusion of an internal investigation which found he bullied and mistreated his staff members. Alison Antes, PhD, co-director and coach of The Professional Skills for Researchers Coaching Program, was invited to write an Op-Ed for the Scientific American newsletter. In the February 11th article How to Be a Great Leader in Science, Antes states that “building a positive research environment requires intention, support and a belief that kindness isn’t weakness.” This article, written in an easy-to-read, digestible fashion, provides eight (8) practical tips to foster and maintain good leadership practices.

BRC’S DR. TRISTAN MCINTOSH FEATURED IN JMR PODCAST
As part of the Greenwall State Medical Boards project, Dr. Tristan McIntosh appeared on the Journal of Medical Regulation (JMR) podcast to discuss her article, “Protecting Patients from Egregious Wrongdoing by Physicians: Consensus Recommendations from State Medical Board Members and Staff.” Listen to the episode here.

BRC RECEIVES R25 GRANT FROM NIH FOR 5-YEAR PROJECT
BRC faculty, Drs. Alison Antes and Tristan McIntosh, received funding through the National Institute of Health to support their 5-year project: Program for Advancing Early-Career Researcher Excellence through Leadership and Management Practices.
Traditional scientific training prepares investigators to conduct scientific research but does not adequately prepare early-career investigators to lead and manage their own labs. This project addresses a gap in the training of the biomedical research workforce by providing early-career investigators with the leadership and management skills needed to foster research rigor, reproducibility, and transparency, responsible conduct of research, and supportive, inclusive research environments.
We will apply findings from leadership and management research, utilize novel training and mentoring methods, and engage members of the research community to obtain feedback and encourage their support of the program. We aim to advance diversity by recruiting and fostering the careers of members of historically underrepresented groups in the biomedical sciences and by increasing inclusive leadership and mentoring practices among program scholars.

BRC WELCOMES CHRISTINE BEREITSCHAFT, MSW
Christine Bereitschaft has joined the Bioethics Research Center in the Division of General Medical Sciences as a Clinical Research Coordinator. She earned a bachelor’s degree in psychology from McKendree University and a master’s degree in social work from Saint Louis University. Christine comes to the BRC from the The National Children’s Cancer Society (NCCS) and will be supporting research projects led by Dr. Bryan Sisk.

BRC WELCOMES NEW STUDENT RESEARCH ASSISTANTS
The BRC is pleased to welcome three new student research assistants – Lucas Cruz, Shelbie Fishman, and Rachel Kalbfell. Cruz will be supporting the HVS project, while Fishman and Kalfell will support Greenwall Portals and VACOM projects.
– 2021 –

BRC PROJECT EXAMINES ETHICAL AND PRACTICAL CHALLENGES TO ADOLESCENT EHI ACCESS
The 21st Century Cures Act mandates that hospitals provide patients with access to electronic health information (EHI), including adolescents. In this project funded by the Greenwall Foundation Make a Difference grant, we will (1) identify risks, benefits, motivations, and barriers to EHI access by performing semi-structured interviews with 40 adolescent/parent dyads; (2) discern implementation practices for adolescent EHI access at US pediatric hospitals; (3) facilitate a stakeholder Delphi panel to generate recommendations for EHI implementation practices. This project will generate empirical knowledge of ethical and practical challenges to adolescent EHI access.

BRC WELCOMES JOSEPH GRAILER TO TEAM
Joseph Grailer, EdD, MFA, has joined the BRC as our newest Program Manager. Dr. Grailer will be working to build the training and mentoring program for junior faculty and postdocs, LAMPS: Leadership and Management Practices for Scientists. He has extensive experience in project management and instructional design and has developed educational programming for a wide range of learners. He is a founding partner of the award-winning small business Paperkeet, and has served on the boards and executive committees of several St. Louis area nonprofits and political organizations. Most recently, Dr. Grailer served as a Senior Project Manager where he was responsible for designing, developing, implementing and evaluating the Entrepreneurship for Biomedicine (E4B) Training Program at Washington University.

BRC WELCOMES NEW STUDENT RAs
The BRC is pleased to welcome three new Student Research Assistants for the semester. Alexandra Patierno is supporting the new HVSL project. Cami Keahi supports the QDS, EBCP and APOLLO projects. Zoe Mercado provides support for the HVSL and APOLLO projects.

BRC PROJECT EXAMINES HOW NATIONAL CULTURE AFFECTS PROFESSIONAL DECISION-MAKING IN RESEARCH
BRC faculty, Drs. Alison Antes and Tristan McIntosh, received National Science Foundation (NSF) funding for their 3-year project: Advancing the Use of Professional Decision-Making Strategies in a Culturally Diverse Research Community. This is the first study of its kind exploring in-depth how researchers from East Asia and the United States approach professional challenges in the research workplace. The key aim of the project is to discover how the cultural background of researchers shapes their decision-making strategies. Unearthing shared and distinct approaches to challenges in research settings will inform revisions to responsible conduct of research (RCR) curriculum for scholars at Washington University that focuses on imparting decision strategies. This research will advance the research community’s understanding of cultural influences on researchers’ decision-making and foster a more culturally inclusive approach to RCR education.

Annie Friedrich, PhD, has joined the Bioethics Research Center in the Division of General Medical Sciences as a Staff Scientist. She earned her PhD in health care ethics from Saint Louis University with concentrations in clinical ethics, empirical research methods, and a certificate in university teaching skills. Prior to joining the BRC, she taught undergraduate and graduate-level classes in bioethics at Saint Louis University and completed several empirical projects on communication in pediatric oncology with investigators in the BRC.

DUBOIS NAMED HASTINGS CENTER FELLOW
The BRC is pleased to announce the election of director, James M. DuBois, PhD, DSc, as a Fellow of the Hastings Center. Hastings Center Fellows are a group of individuals of outstanding accomplishment whose work has informed scholarship and public understanding of complex ethical issues in health, health care, science, and technology. Their common distinguishing feature is uncommon insight and impact in areas of critical concern to the Center–how best to understand and manage the inevitable values questions, moral uncertainties, and societal effects that arise as a consequence of advances in the life sciences, the need to improve health and health care for people of all ages, and mitigation of human impact on the natural world.
James M. DuBois, PhD, DSc, is director of the Bioethics Research Center, the Steven J. Bander Professor of Medical Ethics and Professionalism, and professor of psychology and brain sciences at Washington University School of Medicine in St. Louis. He directs the National Institutes of Health-funded Professionalism and Integrity in Research Program, the first program designed to serve researchers following lapses in research compliance or integrity. He is founding co-editor of Narrative Inquiry in Bioethics: A Journal of Qualitative Research. Dr. DuBois has received more than $12 million in grant funding and is principal investigator of numerous ongoing research projects, including studies on ethical concerns raised by ApoL1 genetic testing of kidney donors with recent African ancestry and evidence-based informed consent practices in clinical trials involving people with dementia. His research interests include empirical research on ethical issues related to informed consent, data sharing, organ transplantation, genetic testing, research integrity, and professional misconduct. His scholarly work also addresses mental health research ethics and the assessment of ethics education outcomes.
– 2020 –

BRC RECEIVES NCATS GRANT
Developing Action Plans for Responding to Noncompliance – UL1TR002345: This project addresses a challenge that institutional officials (IOs) face when dealing with cases of serious or continuing noncompliance: Determining what action plan activities would be most effective to change researchers’ behavior. Interviews and a national survey of IOs will identify how to develop effective action plans. A resource guide will be developed for national dissemination to help IOs’ develop better action plans. This research builds upon the BRC team’s experience directing the Professionalism and Integrity in Research Program (P.I. Program), a national remediation training program for researchers referred for noncompliance. BRC co-investigators on this project include Drs. Alison Antes, Tristan McIntosh, and James DuBois (PI William G. Powderly, MD).

BRC RECEIVES NIA RO1 GRANT
Returning Research Results that Indicate Risk of Alzheimer Disease to Healthy Participants in Longitudinal Studies – NIA 1Ro1AG065234-01: This 5-year project will determine the cognitive and psychological impact of returning genetic and imaging results that indicate 5-year risk of developing Alzheimer Disease to healthy older adults enrolled in studies of aging at the Knight Alzheimer Disease Research Center – one of the world’s leading research centers focused on Alzheimer Disease. Jessica Mozersky will lead this project (MPI with Dr. Sarah Hartz, Psychiatry).

BRC RECEIVES NIH/NIMHD RO1 GRANT
Identifying and Exploring Solutions to the Ethical Challenges of ApoL1 Testing of Donors with Recent African Ancestry Through Mixed Methods Research with Stakeholders – NIH/NIMHD 1R01MD014161-01: Blacks in the United States have a higher incidence of end-stage kidney disease than the general population; much, but not all, of this excess risk is attributed to variants in the ApoL1 gene. During this 3-year project, we will conduct surveys and interviews with individuals who are undergoing ApoL1 testing in the context of a larger organ transplantation research study (APOLLO) in order to explore ethical and social issues. We will work with a diverse panel of stakeholders to establish a consensus on key clinical practices surrounding ApoL1 testing in the context of organ transplantation. James DuBois will lead this project (Co-PI with Dr. Sumit Mohan, Pathology and Immunology).

Elena Kraus, MD, PhD – postdoctoral research fellow through the MTPCI. Elena is an OB/GYN resident who plans to study high-risk women’s perceptions and identify influences on their decision-making regarding reproduction as well as the highly complex healthcare decisions they face during pregnancy.

BRYAN SISK, MD, TO JOIN THE BRC AGAIN
Bryan Sisk, MD, who has served as a Fellow with the BRC, has accepted the position of Assistant Professor in Pediatric Hemoncology and the Bioethics Research Center starting July 1st. Read more about Dr. Sisk HERE.
– 2019 –

Over the past 5 years, BRC faculty have developed and validated six original tests and measures of attitudes, decision-making, knowledge, work habits, and values related to bioethics issues and research ethics. BRC will offer testing services using both fee-for-service and no-cost self-serve models.

The U.S. Office of Research Integrity awarded a grant to the P.I. Program to expand its recruitment and assessment efforts. New assessments will examine the work habits of participants. The project builds on work from the recent NIH K01 Award received by Dr. Alison Antes, the P.I. Program’s most recent faculty member, which includes studying the work habits of research exemplars. Drs. Antes and DuBois recently interviewed 52 researchers who conduct high impact research and enjoy a reputation for great leadership and integrity in research. Data from these projects will inform recommendations on best practices for lab leadership and management.

The BRC received funding from the Institute of Clinical and Translational Sciences (ICTS) to conduct a survey of 400 clinical research coordinators to determine how CRCs learn good clinical practice, or GCP. We will validate a 35-item test of GCP knowledge, inventory their learning experiences including CITI and ACRP online modules, their motivation to learn, and work experiences, and identify the correlates of GCP knowledge.

The Bioethics Research Center (BRC) has launched an education program designed specifically for healthcare providers. BRC faculty and staff are available to facilitate narrative ethics discussion groups, in which participants discuss stories published in the journal Narrative Inquiry in Bioethics. NIB publishes personal stories from patients, family members, and healthcare professionals on socially important topics in healthcare. Discussion group topics currently include: (1) Exploring Stigma and Bias in the Care of Patients; (2) Exploring Moral Distress: Stories from Healthcare Providers; and (3) Communicating What Matters Most to Patients. The BRC anticipates adding new topics on an ongoing basis.
These events are made possible through the support of The Dr. Daniel Bisno Ethics in Medicine Fund.
To request a narrative discussion group session, please complete the request form.
The handouts, containing story excerpts organized around a particular topic, are also available for request by persons interested in using them for educational purposes.
– 2016 –

Dr. Alison Antes, Assistant Professor in the Division of General Medical Sciences at Washington University School of Medicine, received a K01 career development grant from the National Human Genome Research Institute. This grant will support the research program described below which focuses on the management and leadership practices of researchers as they relate to integrity and societal impact in genomic research. The grant will also allow Dr. Antes to receive mentoring and education in genetics, bioethics, and the ethical, legal, and social implications of genomics. Her mentors include Dr. James DuBois, the Steven J. Bander Professor of Medical Ethics and Professionalism at Washington University School of Medicine, and Dr. Laura Bierut, the Alumni Endowed Professor of Psychiatry at Washington University School of Medicine.
Researchers conducting genetic and genomic science play an essential role in advancing biomedical science and its translation and dissemination in medicine. In performing their work, researchers encounter a number of practical ethical, legal, and social challenges, such as research compliance, data integrity, and engaging the public. Moreover, there are significant ethical, legal, and social implications of the outcomes of this research. The purpose of this research is to understand the challenges and needs of genomic researchers with regard to these issues focusing in particular on management practices and leadership practices employed by researchers in navigating issues of research integrity and the societal impact of their work.
In the first phase of this research, we will conduct semi-structured interviews with a national sample of federally-funded researchers nominated as exemplars of professionalism and integrity to identify the management and leadership practices they employ to foster integrity and impact in their research. In a second phase, we will survey a national sample of genomic scientists regarding the management and leadership practices identified in the interviews. We will ask researchers to report on the management and leadership practices they utilize, their confidence in performing each practice, and their interest in training or support materials. In the third phase of this research, we will conduct focus groups with researchers to understand their preferences regarding types of programs and messages about tailored management and leadership programs for scientists. Next, we will develop messages and test their appeal among with researchers using a survey. These findings will facilitate effective messaging about future initiatives to encourage the participation of researchers.
This research will lay the groundwork for future research and practical resources and tools to assist researchers with meeting the various ethical, legal, and social demands of their work, thereby contributing to the quality, integrity, and social impact of genomic science.
– 2014 –

BRC TO EXAMINE ROLE OF CULTURE AND EXPERIENCE ON THE PERCEPTION AND APPLICATION OF RESEARCH REGULATIONS, NORMS, AND VALUES
The BRC received a grant award from the U.S. Office of Research Integrity to examine “the role of culture and experience on the perception of and application of research regulations, norms, and values.” This project builds upon an ongoing ORI-funded project to validate the Professional Decision-making in Research (PDR) and How I Think about Research (HIT-Res) measures. The project will run from August 1, 2014 through July 31, 2016.
Project Title: The role of culture and experience in the perception and application of research regulations, norms and values
Principal Investigator: James M. DuBois, DSc, PhD: Division of General Medical Sciences; Washington University School of Medicine; Campus Box 8504; 660 South Euclid Avenue; St. Louis, MO 63110-1093. Phone 314-747-2710. jdubois@wustl.edu
Co-Investigators: Alison Antes, PhD, Division of General Medical Sciences, Washington University School of Medicine; Tammy English, PhD, Department of Psychology, Washington University in St. Louis.
– 2013 –

The Health Improvement Institute bestowed is Annual Award for Innovation on the Professionalism and Integrity in Research Program for its “P.I. Program Workshop”.
Awards are given for demonstrated excellence in promoting the well-being of people who participate in research. Judges are drawn from academic, compliance, consulting, health services, legal, and research review organizations.

The BRC received a grant from NIH to examine preventing ethical disasters in the practice of medicine.

